Frequently Asked Questions (FAQ)
Sepahan Battery Industrial Complex (SBIC) helps customers to fully identify fundamental information about batteries before selecting and purchasing. In this regard, in the following, the questions are listed that are usually asked about the information that guarantees a suitable and cost-effective purchase.
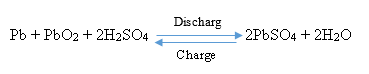
WHAT IS A LEAD-ACID BATTERY?
The type of batteries consists of lead (Pb) as the negative electrode, lead dioxide (PbO2) as the positive electrode immersing in a solution of sulfuric acid as an electrolyte. The overall reaction of charge and discharge in this type of battery is:
WHAT ARE THE MAJOR TYPES OF LEAD-ACID BATTERIES?
We can divide batteries in many ways but mainly by application (what they are used for) and by construction (how they are built). The major applications are Automotive (SLI, micro-hybrid, and hybrid), stationary, motive power (traction), and special applications like submarines and military equipment.
The major construction types are vented (flooded) and VRLA (gel and AGM) batteries.
HOW TO CONNECT A BATTERY IN SERIES?
The positive terminal of the first battery must be connected to the negative terminal of the second battery, the positive terminal of the second must be connected to the negative of the third, and so on. The voltage of the assembled battery is the sum of the battery voltages of the individual batteries. So the connection of batteries is: + to – to + to – to + to -…
WHAT DOES VRLA MEAN?
VRLA is an abbreviation of Valve-regulated lead-acid. AGM and gel are the two kinds of VRLA batteries. These batteries have no “free” liquid electrolyte and in the cell operate on the oxygen recombination cycle, which is designed to reduce water loss. VRLA batteries utilize valves that have a one-way, pressure-relief design.
WHAT ARE THE MAJOR CAUSES OF CAR BATTERY FAILURE?
High temperatures, vibration, Deep discharge, or failure to recharge after voltage drop can cause plate sulfation, and a faulty alternator causes an undercharged or completely discharged battery.
WHAT IS THE SELF-DISCHARGE RATE?
The rate of capacity loss during the storage of the battery is called the self-discharge rate. It is mostly affected by the manufacturer’s technology, raw materials, and condition of battery storage. The self-discharge rate is one of the important factors for measuring lead-acid battery performance.
WHAT IS THE BATTERY CAPACITY?
The battery capacity is the number of Ampere hours (Ah) that the battery will deliver at a specific discharge rate and temperature. It is not a constant value and is seen to decrease with an increased discharge rate. It is affected by a number of elements, for example, active material weight, density of the active material, number, design, and dimensions of plates, discharge rate, adhesion of the active material to the grid, design of separators, specific gravity, and quantity of available electrolyte, plate spacing, grid alloys, final limiting voltage, age, temperature, internal and external resistance, and life history of the battery.
WHAT CAUSES BATTERY HEAT DURING CHARGING?
During the process of battery charging, a large amount of electrical energy transforms into chemical energy. As a charging battery nears full charge, its terminal voltage rises and the charging current falls – depending on the kind of charger which is used. After the battery has reached a full state of charge, additional charging will generate heat, and some water is consumed by electrolysis causing hydrogen and oxygen gasses which be released from the battery. The extra heat is damaging to the plates and separators of the battery, while the loss of water decreases the electrolyte level and increases the specific gravity of the electrolyte. All of these elements can result in shorter battery life.
WHAT IS COLD CRANKING AMPERE (CCA)?
CCA is a rating that is used in the battery industry to define a battery’s ability to start an engine in cold temperatures. The rating refers to the number of amps a 12-volt battery can deliver at 0°F for 10 or 30 seconds (depending on the test method). The Cold Cranking Ampere (CCA) current is declared by the manufacturer and can be denoted by European standard (EN) International Electro-technical Commission standard (IEC) and German standard (DIN).
WHAT IS BATTERY RESERVE CAPACITY (RC)?
Reserve Capacity (RC) is the number of minutes (min) a fully charged battery at the specific temperature will discharge 25 amps until the battery voltage drops below 10.5 volts. Reserve Capacity is a general indicator of how long a new and fully charged battery can continue to operate essential accessories if the vehicle’s alternator fails.
WHAT IS STARTING, LIGHTING, IGNITION (SLI) BATTERY?
These kinds of batteries deliver a large amount of power for a short time as needed for normal engine starting. The battery is then recharged by the alternator. Starting batteries are not designed to withstand multiple discharge/recharge cycles, unlike deep-cycle batteries.
WHAT IS THE VOLTAGE OF A BATTERY?
Voltage refers to the amount of electrical potential that a battery holds. The standard automotive battery in today’s cars is a 12-volt battery. Each battery has six cells, each with 2.1 volts at full charge. A car battery at 12.6 volts or higher is considered fully charged.
WHAT POINTS SHOULD BE CONSIDERED IN THE BATTERY INSTALLATION?
A positive terminal should be connected first and a negative one should be connected later when installing the battery.
WHAT DOES 12 V 60 AH 560 A ON THE LABEL OF THE BATTERY MEAN?
The value 12V on the label shows the electrical voltage of the battery in volts. The value 60 Ah indicates the battery capacity. The value 560A indicates the cold cranking ampere to be provided by the battery at -18˚C.
HOW LONG WILL A BATTERY LAST?
The lifetime of a battery can be different by how it is used, maintained, charged temperature, and other elements.
WHAT IS A GEL BATTERY?
Gel battery is a type of VRLA lead-acid battery in which electrolyte is immobilized in the gel using a gelling agent. This type of battery has oxygen recombination to reduce water loss and gassing. “Gel” batteries are non-spillable even if they are broken. They must be charged at a lower voltage than flooded batteries to prevent extra gassing and pressure from increasing inside the cells.
CAN BATTERIES FREEZE?
The electrolyte in a lead-acid battery may freeze in a partially discharged state. At a 40% state of charge, the electrolyte will freeze if the temperature reaches approximately 16.0°F. The freezing temperature of the electrolyte in a fully charged battery is -92.0°F.
DO WE EVER NEED TO ADD ACID TO OUR BATTERY?
You never need to add acid under normal conditions. For a standard auto, only distilled, deionized, or approved water should be added to achieve the recommended levels mentioned above. When a battery is shipped in a dry state or accidental spillage occurs, electrolytes should be added to the battery. Once filled, it may only need water addition.
DO BATTERIES SELF-DISCHARGE WHEN NOT IN USE?
All batteries, regardless of their chemistry, self-discharge. The rate of self-discharge depends on the battery type and the temperature of storage. The rate of self-discharge for maintenance-free lead-acid batteries is about 3% per month at 80°F.
IS THERE A MAXIMUM TEMPERATURE FOR CHARGING LEAD-ACID BATTERIES?
When charging lead-acid batteries, the temperature should not exceed 120°F. In this condition, the battery should be taken off charge and allowed to cool before resuming the charging process.
ARE LEAD-ACID BATTERIES RECYCLABLE?
These batteries are 100 percent recyclable. Lead is the most recycled metal in the world. The plastic containers and covers of old batteries are neutralized, reground, and used in the manufacture of new battery cases. The electrolyte can be processed for recycled wastewater uses. In some cases, the electrolyte is cleaned reprocessed and sold as a battery-grade electrolyte. In other cases, the sulfate content is removed as Ammonia Sulfate and used in fertilizers. The separators are often used as a fuel source for the recycling process.
WHAT IS A MAINTENANCE-FREE (MF) BATTERY?
A Maintenance-Free battery is a type of battery that normally requires no service watering during its lifetime of use.


